Welcome to the Winn Research Lab
Lung cancer remains the leading cause of cancer-related deaths worldwide, with non-small cell lung cancer (NSCLC) accounting for approximately 90% of all diagnosed cases. Despite advancements in surgery, chemotherapy, and radiotherapy, the five-year survival rate for lung cancer patients remains below 27%. Smoking continues to be a major contributor to lung cancer, with over half of those diagnosed being former smokers. Even with the implementation of smoking cessation programs, the lifetime risk of developing lung cancer among former smokers remains significantly elevated. This highlights the urgent need for more effective and innovative molecular therapies targeting this high-risk population.
The Winn Lab focuses on investigating the molecular mechanisms and signaling pathways by which oncogenes and tumor suppressors drive the initiation and progression of lung cancer, utilizing both human and animal models. Through advanced, cutting-edge technologies, the lab conducts translational research to discover novel biomarkers that can inform the development of innovative therapeutic approaches.
In addition to its focus on fundamental cancer biology, the Winn Lab is also committed to investigating lung cancer health disparities. This includes exploring genetic, proteomic, and metabolomic variations across different populations to better understand differences in survival outcomes.
Supported by funding from the NIH and the Department of Veterans Affairs, the Winn Lab maintains a robust network of national and international collaborations to further its mission of advancing lung cancer research and treatment.
Research
Learn more about our cutting edge research
Our Team
Meet our research group

Parash Parajuli, PhD
Assistant Professor


Michelle Van Scoyk
Lab Manager/Senior Lab Technician

Michelle Van Scoyk
Lab Manager/Senior Lab Technician
Massey Cancer Center

Gregory Riddick, PhD
Senior Computational Biologist


Bin Zhu, PhD
Research Computational Biologist


Chu-Fang (Herman) Chou, PhD
Senior Research Scientist

Chu-Fang (Herman) Chou, PhD
Senior Research Scientist
Massey Cancer Center
Email: chufang.chou@vcuhealth.org

Pei-Ying (Maggie) Wu
Senior Lab Technician


Stephanie McHale
Lab Specialist (In-vivo models)

Publications
Learn more about our work
Dr. Winn’s complete bibliography at MyNCBI:
https://www.ncbi.nlm.nih.gov/myncbi/robert.winn.1/bibliography/public/
Contacts
Winn Research Lab
Michelle Van Scoyk
Lab Manager/Senior Research Scientist
Massey Cancer Center
Phone: (804) 827-1158
Email: michelle.vanscoyk@vcuhealth.org
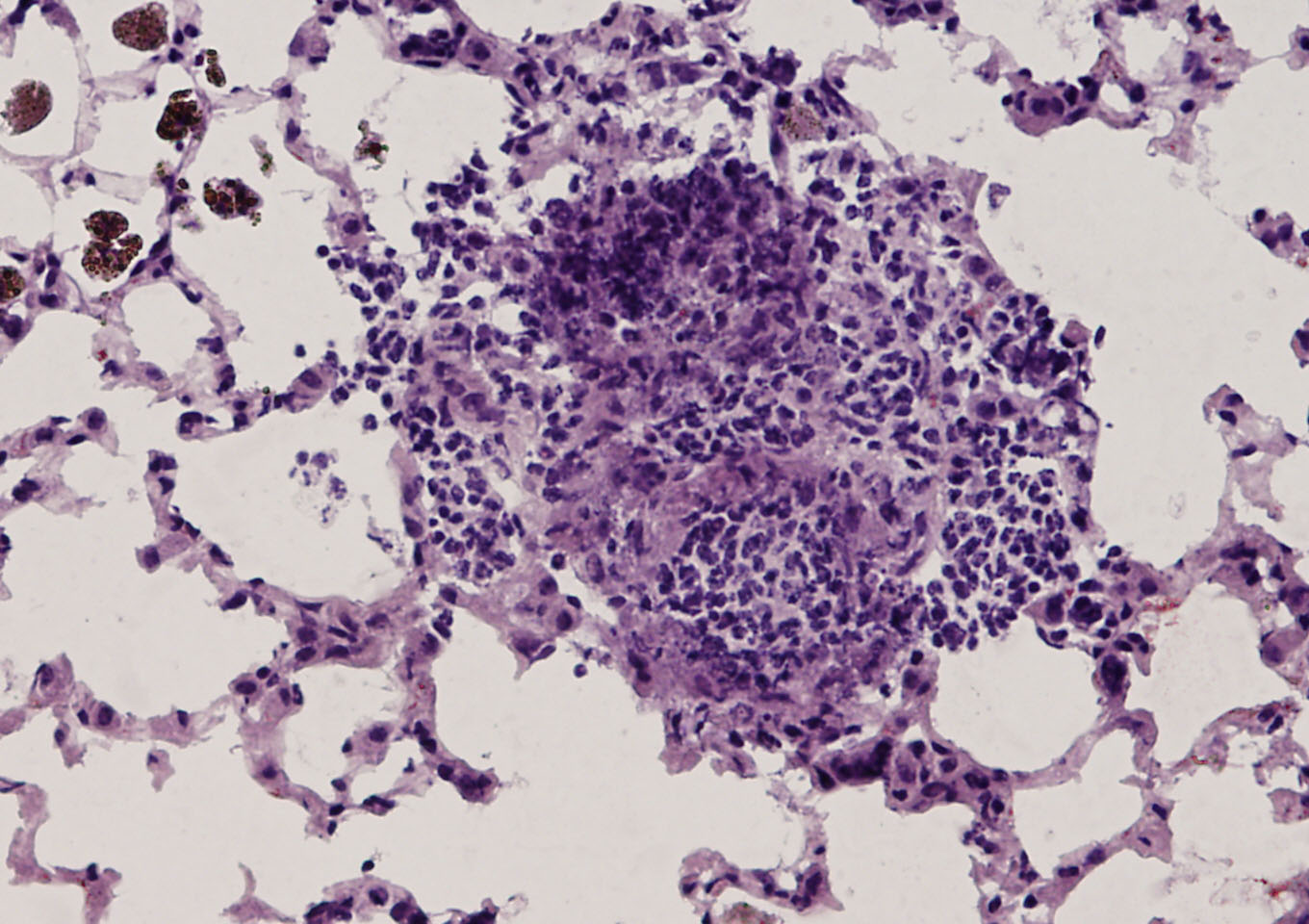
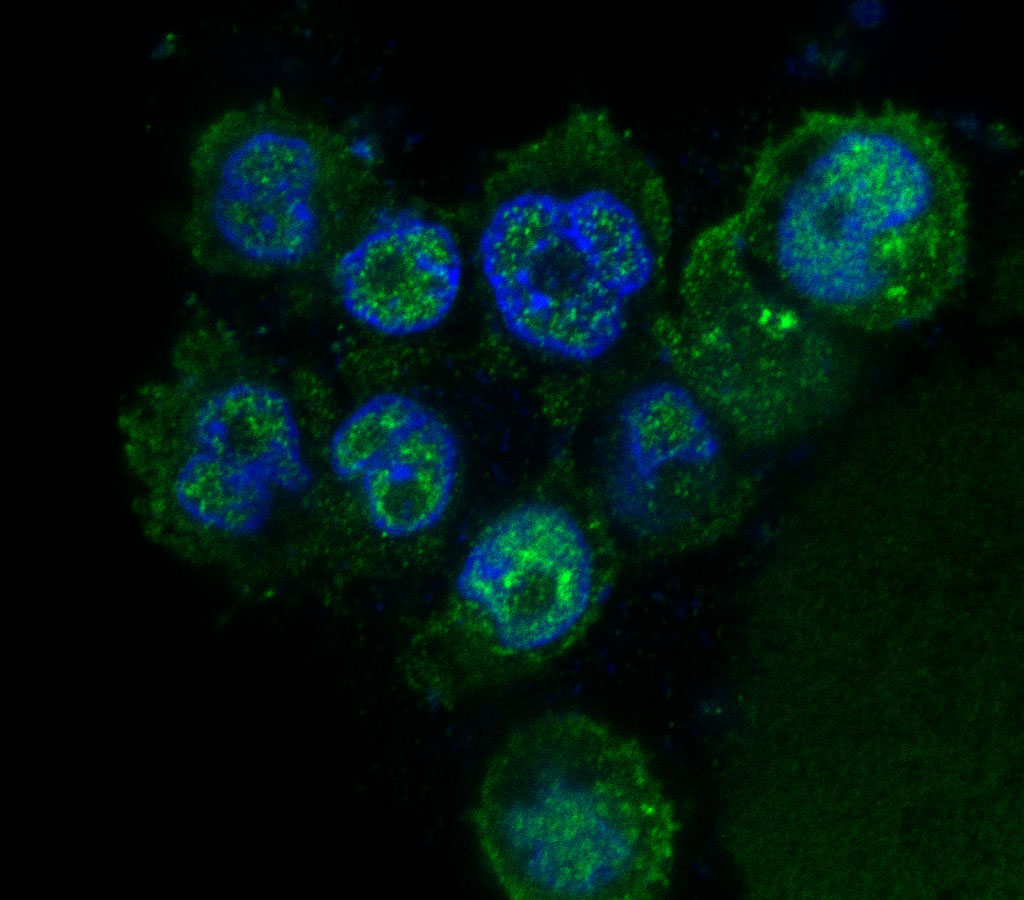
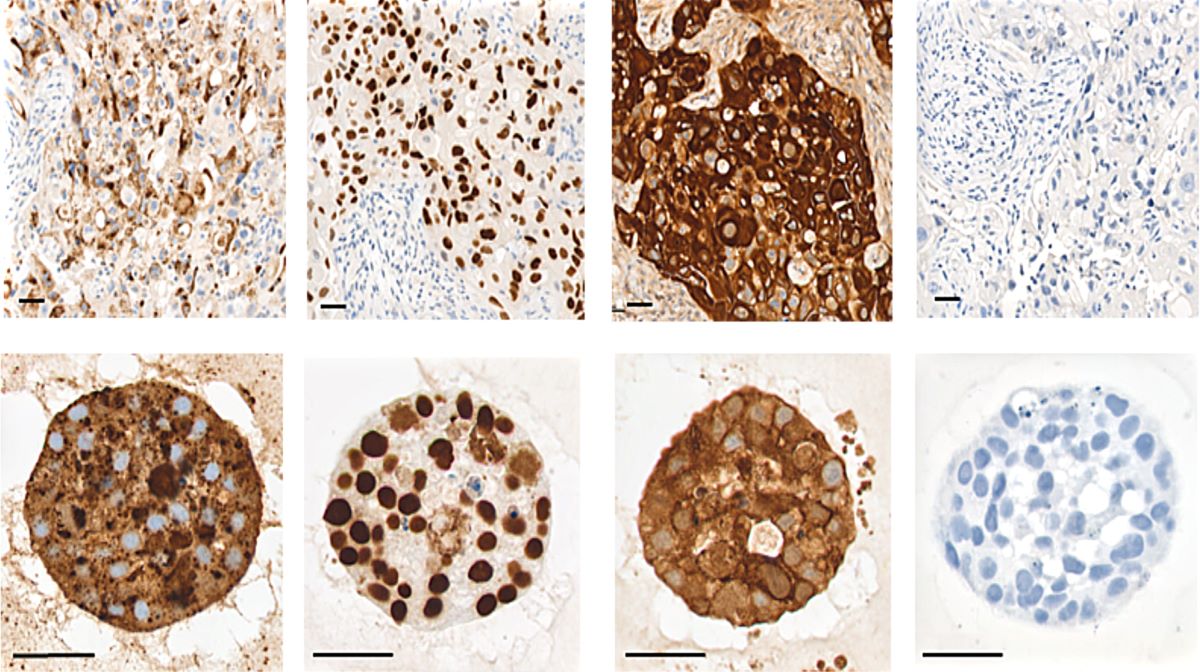
 Elevated levels of protein arginine methyltransferases (PRMTs) have been associated with poorer prognosis across various cancer types. In our study, we found that PRMT6 is more highly expressed in lung cancer tissues from Black/African-American men compared to non-Hispanic White men. We explored the molecular mechanism behind this observation and discovered that PRMT6 collaborates with PRMT1 to form a heteromeric complex that promotes lung cancer progression. Targeting this PRMT1/PRMT6 heteromer using a competitive peptide effectively reduced cell proliferation in non-small cell lung cancer (NSCLC) cell lines and patient-derived organoids. These findings highlight a promising, more tailored therapeutic strategy for Black/African- American men with lung cancer and contribute to efforts aimed at addressing cancer health disparities.
Elevated levels of protein arginine methyltransferases (PRMTs) have been associated with poorer prognosis across various cancer types. In our study, we found that PRMT6 is more highly expressed in lung cancer tissues from Black/African-American men compared to non-Hispanic White men. We explored the molecular mechanism behind this observation and discovered that PRMT6 collaborates with PRMT1 to form a heteromeric complex that promotes lung cancer progression. Targeting this PRMT1/PRMT6 heteromer using a competitive peptide effectively reduced cell proliferation in non-small cell lung cancer (NSCLC) cell lines and patient-derived organoids. These findings highlight a promising, more tailored therapeutic strategy for Black/African- American men with lung cancer and contribute to efforts aimed at addressing cancer health disparities.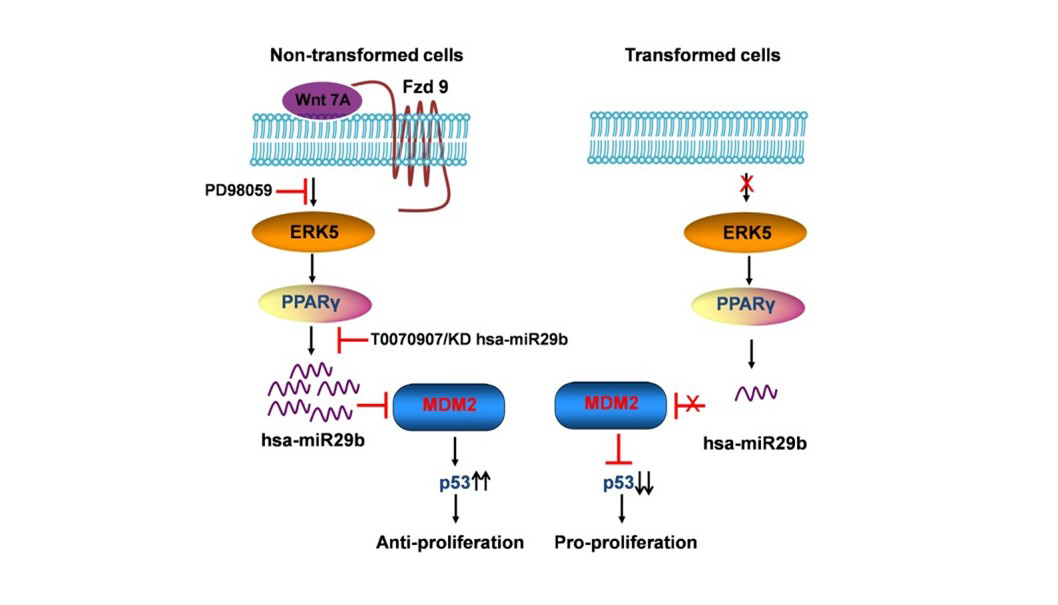 The dysregulation of the canonical and non-canonical Wnt signaling pathways has been a common theme in many types of cancers, most notably mediated by the stabilization of β-catenin in the canonical pathway to drive tumor progression. Several Wnts have been shown to be upregulated in Non-Small Cell Lung Cancer (NSCLC) tissue and cell lines. However, we were the first to identify that Wnt7A was downregulated in lung cancer and played a critical role in the maintenance of normal epithelial phenotype, independent of β-catenin. It is through a seven-transmembrane receptor, Frizzled-9, that Wnt7A reverses epithelial-mesenchymal-transition and transformed cell growth, re-establishes cellular polarity, and targets downstream tumor suppressor genes such as PPAR-gamma and Sprouty-4. We have also identified that Wnt7A is methylated in a subset of lung tumors and involved in cellular arrest through the targeting of genes involved in the senescence pathway. We continue to expand our research on this novel role of Wnt7A and explore its value as a possible future therapeutic approach for the treatment of lung cancer. Towards this end, we are currently testing the efficacy of Wnt7A in reducing lung cancer progression using the form of a small molecular nanoliposome in our in vivo lung tumor models and lung cancer patient-derived organoids.
The dysregulation of the canonical and non-canonical Wnt signaling pathways has been a common theme in many types of cancers, most notably mediated by the stabilization of β-catenin in the canonical pathway to drive tumor progression. Several Wnts have been shown to be upregulated in Non-Small Cell Lung Cancer (NSCLC) tissue and cell lines. However, we were the first to identify that Wnt7A was downregulated in lung cancer and played a critical role in the maintenance of normal epithelial phenotype, independent of β-catenin. It is through a seven-transmembrane receptor, Frizzled-9, that Wnt7A reverses epithelial-mesenchymal-transition and transformed cell growth, re-establishes cellular polarity, and targets downstream tumor suppressor genes such as PPAR-gamma and Sprouty-4. We have also identified that Wnt7A is methylated in a subset of lung tumors and involved in cellular arrest through the targeting of genes involved in the senescence pathway. We continue to expand our research on this novel role of Wnt7A and explore its value as a possible future therapeutic approach for the treatment of lung cancer. Towards this end, we are currently testing the efficacy of Wnt7A in reducing lung cancer progression using the form of a small molecular nanoliposome in our in vivo lung tumor models and lung cancer patient-derived organoids.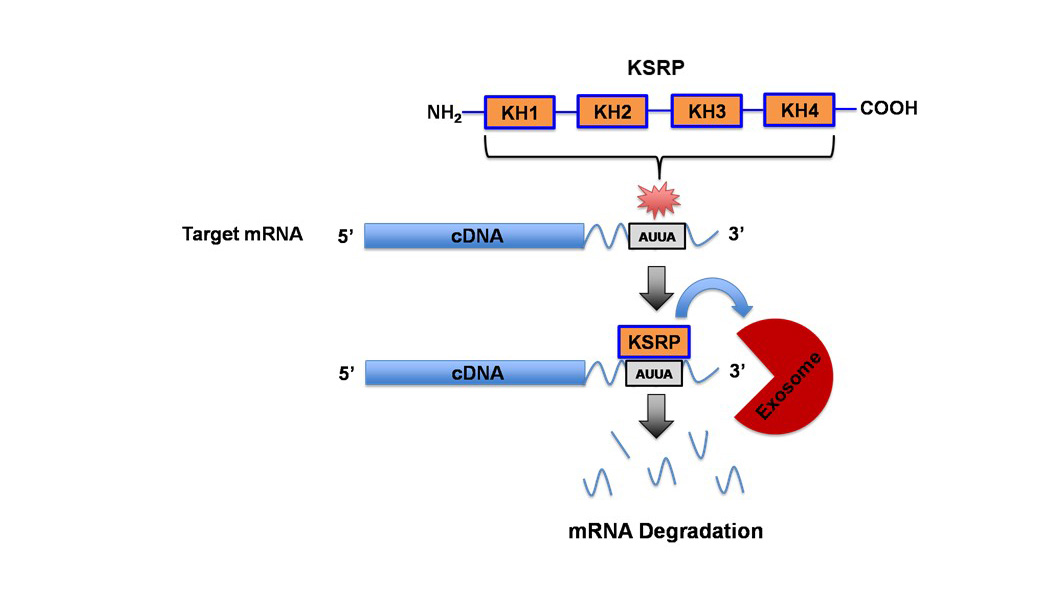 AU-rich elements are found in transcripts encoding proteins that regulate inflammation, transcription, cell proliferation, RNA metabolism, development, and cellular signaling; all of which play important roles in tumorigenesis. KSRP is an AU-rich element RNA binding protein that specifically binds and recruit's RNA decay machinery to ARE-containing transcripts, thereby, targeting them to degradation. Our laboratory was the first to define a novel role for KSRP in lung cancer. It is robustly upregulated in lung cancer patient samples and promotes cell proliferation, migration/invasion, and transformed cell growth in NSCLC cell lines. We have demonstrated that mutations in the KRAS oncogene results in upregulation of KSRP and that deletion of KSRP reduces lung tumor burden in a KRAS mutation-induced lung cancer model. Our goals for this project are: 1) elucidate the mechanisms of KSRP upregulation by mutant KRAS, 2) identify KSRP targets in lung cancer and 3) identify small molecule inhibitors of KSRP. These studies will lead to potential therapeutics for lung cancer patients with KRAS mutations by inhibiting the functions of KSRP.
AU-rich elements are found in transcripts encoding proteins that regulate inflammation, transcription, cell proliferation, RNA metabolism, development, and cellular signaling; all of which play important roles in tumorigenesis. KSRP is an AU-rich element RNA binding protein that specifically binds and recruit's RNA decay machinery to ARE-containing transcripts, thereby, targeting them to degradation. Our laboratory was the first to define a novel role for KSRP in lung cancer. It is robustly upregulated in lung cancer patient samples and promotes cell proliferation, migration/invasion, and transformed cell growth in NSCLC cell lines. We have demonstrated that mutations in the KRAS oncogene results in upregulation of KSRP and that deletion of KSRP reduces lung tumor burden in a KRAS mutation-induced lung cancer model. Our goals for this project are: 1) elucidate the mechanisms of KSRP upregulation by mutant KRAS, 2) identify KSRP targets in lung cancer and 3) identify small molecule inhibitors of KSRP. These studies will lead to potential therapeutics for lung cancer patients with KRAS mutations by inhibiting the functions of KSRP.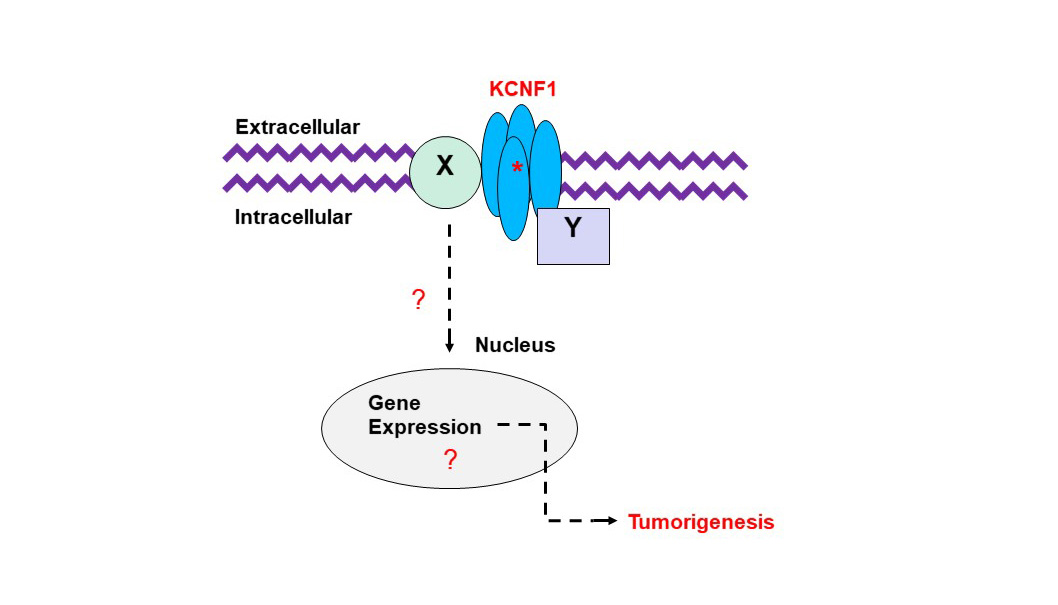 Voltage-gated potassium (Kv) channels regulate cell excitability and cell proliferation. Expression of Kv channels is dysregulated in many types of tumors including lung cancer, leading to the interest of targeting them in cancer patients. Ion channels have been extensively studied in cancer biology where the potassium ion channel family has long been known to have altered expression in lung tumors. However, past attempts of treating cancer with various potassium ion channel-blocking agents have proven to be disappointing, as most of these inhibitors directly or indirectly affect the electrophysiological properties of excitable cells, e.g. myocytes and/or neurons, which are crucial to the functions of the cardiac and nervous systems. Our laboratory discovered an increased expression of a unique “electrically silent” Kv channel, (KCNF1), in lung cancer. We demonstrated that KCNF1 drives transformed cell growth and is important to maintain the basement membrane integrity of NSCLC cells. Our goals for this research are: 1) identify the mechanism of how KCNF1 functions in the lung, 2) identify KCNF1 targets in lung cancer and 3) identify small molecule inhibitors of KCNF1. These studies will lead to potential therapeutics for lung cancer patients by inhibiting the functions of KCNF1.
Voltage-gated potassium (Kv) channels regulate cell excitability and cell proliferation. Expression of Kv channels is dysregulated in many types of tumors including lung cancer, leading to the interest of targeting them in cancer patients. Ion channels have been extensively studied in cancer biology where the potassium ion channel family has long been known to have altered expression in lung tumors. However, past attempts of treating cancer with various potassium ion channel-blocking agents have proven to be disappointing, as most of these inhibitors directly or indirectly affect the electrophysiological properties of excitable cells, e.g. myocytes and/or neurons, which are crucial to the functions of the cardiac and nervous systems. Our laboratory discovered an increased expression of a unique “electrically silent” Kv channel, (KCNF1), in lung cancer. We demonstrated that KCNF1 drives transformed cell growth and is important to maintain the basement membrane integrity of NSCLC cells. Our goals for this research are: 1) identify the mechanism of how KCNF1 functions in the lung, 2) identify KCNF1 targets in lung cancer and 3) identify small molecule inhibitors of KCNF1. These studies will lead to potential therapeutics for lung cancer patients by inhibiting the functions of KCNF1.